Nazish Parveen, Sajid Ali Ansari
1 Department of Chemistry, College of Science, King Faisal University, P.O. Box 380, Hofuf, Al-Ahsa 31982, Saudi Arabia
2 Department of Physics, College of Science, King Faisal University, P.O. Box 400, Hofuf, Al-Ahsa 31982, Saudi Arabia
* Author to whom correspondence should be addressed:
nislam@kfu.edu.sa (N. Parveen)
ABSTRACT
Considerable attention has been directed towards the development of manganese oxide materials capable of simultaneously offering ample surface area and facilitating charge movement to enhance capacitance performance. In this study, oval-shaped manganese oxide (OS-MO) was synthesized via a straightforward hydrothermal method, followed by comprehensive characterization using various microscopic and spectroscopic techniques. The electrochemical supercapacitive performance of the resulting OS-MO-based electrode was evaluated in a three-electrode assembly workstation employing cyclic voltammetry and galvanostatic charge-discharge methods. The experimental findings revealed that OS-MO achieved an impressive specific capacitance of 629.62 Fg-1. Furthermore, the cyclic stability of OS-MO remained consistently high at approximately 89% over 1000 cycles at a fixed current density. These results suggest that the superior performance and stability of OS-MO can be attributed to its porous nature and high surface area. Consequently, OS-MO emerges as a promising candidate for applications in energy storage systems.
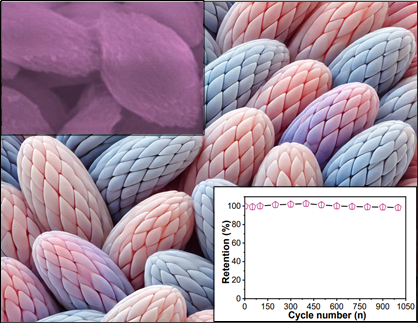
Significance of the study:
This study highlights the development of oval-shaped manganese oxide (OS-MO) electrodes with high specific capacitance and stability, advancing the field of supercapacitors for efficient and sustainable energy storage solutions.
Summary of the study:
The research presents a hydrothermal synthesis of OS-MO, achieving a specific capacitance of 629.62 F/g and 89% retention over 1,000 cycles, showcasing its potential for high-performance energy storage applications due to its porous nature and high surface area.
