Babasaheb T. Shinde, Santosh B. Babar, Umesh V. Shembade, Hemant V. Chavan
1 Department of Chemistry, A.S.P. College (Autonomous), Devrukh, Dist. Ratnagiri-415 804, Maharashtra. India.
2 Department of Chemistry, S.K.S.V. College, Lanja, Dist. Ratnagiri, India
3Regional Forensic Science Laboratory, Kolhapur-416006, India
4Thin Film Nano Material Laboratory, Department of Physics, Shivaji University Kolhapur-416004, Maharashtra, India.
*Author to whom correspondence should be addressed:
hemantchavan.sus@rediffmail.com (Hemant V. Chavan)
ABSTRACT
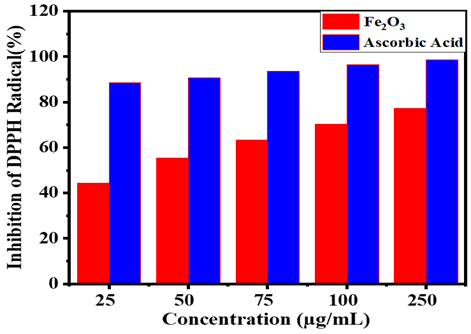
Iron oxide (Fe₂O₃) nanoparticles were successfully synthesized from naturally abundant red soil using an eco-friendly magnetic separation method and evaluated for their antimicrobial and antioxidant properties. The nanoparticles were characterized using X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), and field-emission scanning electron microscopy (FE-SEM), confirming their crystalline hematite phase, functional groups, and morphological features. The antibacterial efficacy was tested against Gram-positive (Bacillus subtilis, Bacillus cereus) and Gram-negative (Escherichia coli, Proteus vulgaris) bacteria, while antifungal activity was assessed against Candida albicans. The results demonstrated significant antimicrobial activity, with inhibition zones comparable to standard antibiotics (streptomycin and ketoconazole). Additionally, the antioxidant potential was evaluated using the DPPH radical scavenging assay, revealing substantial free radical inhibition (up to 77.32% at 250 µg/mL), though slightly lower than ascorbic acid. The study highlights the dual functionality of Fe₂O₃ nanoparticles as both antimicrobial and antioxidant agents, synthesized through a sustainable, low-cost, and environmentally benign approach. This method eliminates the need for toxic chemicals, reduces energy consumption, and leverages naturally occurring iron-rich soil, making it a viable alternative to conventional nanoparticle synthesis techniques. The findings underscore the potential of Fe₂O₃ nanoparticles in biomedical applications, particularly in combating antibiotic-resistant infections and oxidative stress-related diseases, while aligning with green chemistry principles for sustainable nanotechnology development.
Significance of the Study:
The study presents an environmentally benign, cost-effective approach for nanoparticle synthesis, reducing reliance on toxic chemicals. The demonstrated antimicrobial and antioxidant properties of Fe₂O₃ nanoparticles offer promising solutions for combating antibiotic resistance and oxidative stress-related diseases. Their natural sourcing and scalable production align with green chemistry principles, making them viable for biomedical and environmental applications. This research contributes to sustainable nanotechnology while addressing global health challenges posed by drug-resistant infections and chronic oxidative damage.
Summary of the Study:
This study demonstrated a sustainable method for synthesizing Fe₂O₃ nanoparticles from iron-rich red soil using magnetic separation and calcination. The nanoparticles exhibited potent antibacterial activity against Gram-positive and Gram-negative bacteria, significant antifungal effects against Candida albicans, and notable antioxidant properties in DPPH assays. Characterization via XRD, FT-IR, and FE-SEM confirmed their crystalline hematite phase, functional groups, and morphology. The eco-friendly synthesis and multifunctional bioactivity highlight their potential as alternatives to conventional antimicrobial and antioxidant agents.
