Devesh, M. P. Singh
Department of Physics, Institute of Basic Science, Dr. Bhimrao Ambedkar University, Khandari Campus, Agra-282002, Uttar Pradesh, India
*Author to whom correspondence should be addressed:
devesh452025@gmail.com (Devesh)
ABSTRACT
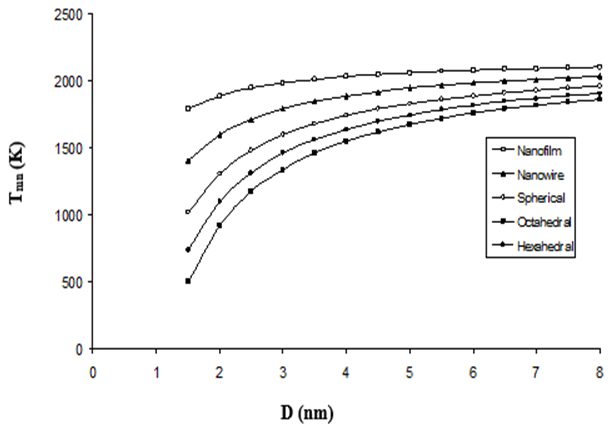
The thermodynamic properties of nanoparticles, particularly their melting temperature, are strongly influenced by size, shape, and surface effects. In this study, we investigate the melting behavior of rutile-phase titanium dioxide (TiO₂) nanoparticles using a modified cohesive energy model that incorporates crystal structure and surface contributions. The model is applied to various nanoparticle shapes, including spherical, nanowire, nanofilm, octahedral, and hexahedral geometries, to assess their impact on melting temperature. Our results demonstrate a significant size-dependent reduction in melting temperature for nanoparticles in the 2–12 nm range, consistent with prior theoretical and molecular dynamics (MD) simulation studies. The cohesive energy model is refined to account for surface atom contributions, shape factors, and atomic packing efficiency, providing a robust framework for predicting melting behavior. Comparisons with existing MD simulations and Buffat-Borel model predictions reveal close agreement for larger nanoparticles (>6 nm), while slight deviations occur for smaller nanoparticles (<6 nm), likely due to enhanced edge and corner effects. Notably, the melting temperature decreases more rapidly for nanoparticles with higher shape factors, such as hexahedral and octahedral structures, compared to spherical nanoparticles of equivalent volume. In the absence of extensive experimental data, our theoretical predictions offer valuable insights into the thermodynamic stability of TiO₂ nanoparticles, which are crucial for applications in photocatalysis, photovoltaics, and nanotechnology. The findings highlight the importance of shape and size control in tailoring the thermal properties of nanomaterials for specific technological applications.
Significance of the Study:
This study provides critical insights into the size- and shape-dependent melting behavior of rutile TiO₂ nanoparticles, essential for high-temperature applications in catalysis, coatings, and electronics. By refining the cohesive energy model to incorporate surface and geometric effects, it offers a computationally efficient alternative to molecular dynamics simulations. The findings reveal that sub-6 nm nanoparticles, especially non-spherical ones, exhibit significantly reduced melting points, guiding the design of thermally stable nanomaterials for advanced technologies where precise thermal control is crucial.
Summary of the Study:
This study employs a cohesive energy model to analyze how size (2–12 nm) and shape (spherical, nanowire, octahedral, etc.) affect rutile TiO₂ nanoparticles’ melting temperature. Results show a sharp decline in melting points below 6 nm, with non-spherical structures (e.g., hexahedral) experiencing the fastest reduction due to higher surface energy. The model agrees well with MD simulations for larger nanoparticles (>6 nm) but highlights edge/corner effects in smaller ones, aiding nanomaterial design for thermal stability in industrial applications.
