Gamze Erdoğdu
İnönü University, Faculty of Arts & Sciences, Department of Chemistry, 44280, Malatya, Turkey
* Author to whom correspondence should be addressed:
gamze.erdogdu@inonu.edu.tr
ABSTRACT
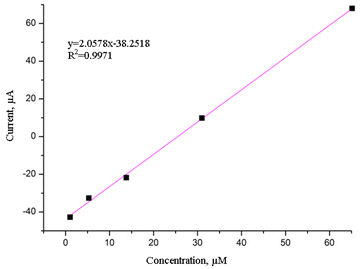
In this study, ferrocene was used to modify a glassy carbon electrode, which was fixed with an appropriate amount of nafion solution. The -C-O-C- and -COOH functional groups were detected in the ferrocene/graphene composite, which indicated that graphene was oxidized to graphene oxide, making it more hydrophilic, dispersible and compatible with polymers, thus increasing the diazinon detection rate. The optimal conditions for diazinon detection were found using cyclic voltammetry (CV): a scan rate of 50 mV/s, pH 6.6, and 0.1 mol/L PBS buffer solution. Differential pulse voltammetry (DPV) shows that the concentrations of diazinon are 1×10-6 – 1.2×10-5 mol/L, and there is a good linear relationship between the peak current and the concentration: Ipa (μA)=2.0578 c(μM) -38.2518, R2 = 0.9971; the detection limit is 1.2×10-9 mol/L. This modified electrode has good stability, repeatability and operability and can be applied to detect actual samples.
Significance of the study:
This study introduces a highly sensitive and stable ferrocene/graphene-modified glassy carbon electrode for detecting diazinon insecticide. By leveraging the synergistic effects of ferrocene and graphene, the research enhances electron transfer and electro-catalytic performance, offering a promising tool for environmental monitoring and broadening the scope for detecting various contaminants.
Summary of the study:
The study developed a ferrocene/graphene-modified glassy carbon electrode for efficient diazinon detection. Using cyclic voltammetry (CV) and differential pulse voltammetry (DPV), the electrode demonstrated high sensitivity, stability, and a broad detection range. The modification significantly improved electron transfer and catalytic activity, showcasing potential applications in environmental monitoring and contaminant detection.
