Narendra Prasad Tripathi, Brijesh K. Pandey, Abhay P. Srivastava
1Department of Chemistry, R. R. Institute of Modern Technology, Lucknow, Uttar Pradesh, India
2Department of Physics and Materials Science, Madan Mohan Malviya University of Technology, Gorakhpur, Uttar Pradesh, India
*Author to whom correspondence should be addressed:
bkppms@mmmut.ac.in (B. K. Pandey)
ABSTRACT
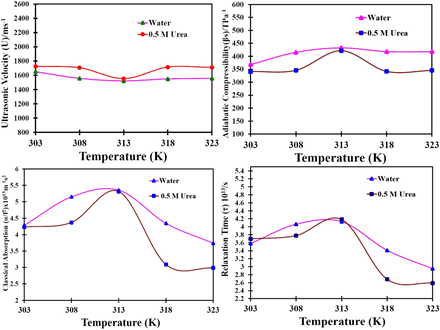
This study investigates the ternary mixture of sodium dodecyl sulphate (SDDS) with urea in water through ultrasonic and thermodynamic analyses at temperatures of 303, 308, 313, 318, and 323 K. Experimental values were used to calculate derived parameters such as ultrasonic velocity (U), density (ρ), and viscosity (η). These parameters enabled the computation of acoustic properties, including adiabatic compressibility (βs) and intermolecular free length (Lf). The findings indicate that urea exhibits hydrophilic (structure-breaking, SB) behavior with weak interactions at lower temperatures (303-308 K) and hydrophobic (structure-making, SM) behavior with stronger interactions at higher temperatures (313-323 K). The study demonstrates that ultrasonic velocimetry is a powerful and precise method for characterizing interactions between surfactants and biomolecules. The temperature coefficients of ultrasonic velocity (∂U/∂T) and adiabatic compressibility (∂βs/∂T) were found to have positive and negative values, respectively, further confirming the temperature-dependent interaction behavior of urea. Additionally, relaxation time analysis revealed that the maximum relaxation time for the binary solution occurs at 312 K and the minimum at 323 K, whereas for the ternary solution, the maximum relaxation time is also at 312 K and the minimum at 320 K. These results have significant implications in fields such as solution chemistry, biochemistry, biosciences, surface science, surfactant chemistry, industrial chemistry, and both physical and chemical sciences.
Significance of the study:
This study explores the molecular interactions in a sodium dodecyl sulphate (SDDS), urea, and water ternary mixture using ultrasonic and thermodynamic analyses at different temperatures. The research reveals that urea transitions from hydrophilic to hydrophobic behavior as temperature increases. These findings enhance our understanding of surfactant-biomolecule interactions, benefiting fields such as solution chemistry, biochemistry, and surfactant chemistry.
Summary of the study:
The study uses ultrasonic velocimetry to investigate the SDDS-urea-water ternary mixture at temperatures from 303 K to 323 K. Key parameters like ultrasonic velocity, density, and viscosity were measured to compute acoustic properties, indicating urea’s hydrophilic behavior at lower temperatures and hydrophobic behavior at higher temperatures. Temperature coefficients of ultrasonic velocity and adiabatic compressibility confirmed the temperature-dependent interaction behavior. Relaxation time analysis showed varying interaction dynamics across temperatures, with significant implications for solution chemistry, biochemistry, and surfactant chemistry.
