Jaiby Joseph, Mercy Mathews, Arpitha Prabhakaran
Kuriakose Elias College Mannanam P.O, Kottayam, India
* Author to whom correspondence should be addressed:
jjoseph@kecollege.ac.in (J. Joseph)
ABSTRACT
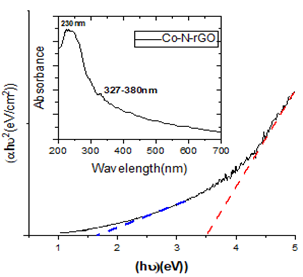
Graphene oxide (GO) and its derivatives have emerged as promising materials for energy storage applications due to their exceptional physical and chemical properties. In this study, nitrogen-doped reduced graphene oxide (N-rGO) and a cobalt-nanocomposite of graphene oxide (Co-N-GO) were synthesized via hydrothermal methods. The structural and compositional characteristics of the resulting materials were confirmed through various analytical techniques, including X-ray diffraction (XRD), Fourier-transform infrared (FTIR) spectroscopy, ultraviolet-visible (UV-Vis) spectroscopy, and Raman spectroscopy. XRD analysis revealed a decrease in the average crystallite size upon nitrogen doping, as determined using the Scherrer equation, indicative of enhanced material dispersion. FTIR spectra provided evidence of successful reduction of GO to rGO and confirmed the presence of C-N bonds, further supporting the doping process. UV-Vis spectroscopy indicated a red shift in absorption peaks towards the visible region, accompanied by a lowering of absorption bands, which suggests the formation of compensating energy states and shifts in conduction band edges due to doping. The bandgap energy, calculated using Tauc plots, showed a significant reduction in N-doped and Co-incorporated samples, highlighting the potential of these materials in enhancing the performance of energy storage devices.
Significance of the study:
This study demonstrates an economical and efficient method for synthesizing nitrogen-doped reduced graphene oxide (N-rGO) and cobalt-nanocomposite graphene oxide (Co-N-GO) for energy storage applications. The materials exhibit improved electrical conductivity, tunable bandgap, and enhanced catalytic activity, making them ideal for advanced energy devices like supercapacitors and fuel cells. This scalable approach offers significant potential for the development of high-performance materials in renewable energy technologies.
Summary of the study:
The study synthesized N-rGO and Co-N-GO using hydrothermal methods, confirmed through XRD, FTIR, UV-Vis, and Raman spectroscopy. Nitrogen doping and cobalt incorporation resulted in improved structural, optical, and electrical properties, with reduced crystallite size and bandgap. These advancements make the materials highly suitable for energy storage and catalytic applications, providing a scalable solution for future energy technologies.
