Shipra Tripathi, Anjani K. Pandey, Kailash Narayan Uttam, C. K. Dixit
1 Department of Physics, Dr. R K Pal Degree College, Kannauj, Uttar Pradesh, India
2 Department of Applied Science and Humanities, Institute of Engineering & Technology, Dr. Shakuntala Misra National Rehabilitation University, Lucknow, Uttar Pradesh, India
3 Saha’s Spectroscopy Laboratory, Department of Physics, University of Allahabad, Prayagraj, India
4 Department of Physics, Faculty of Science & Technology, Dr. Shakuntala Mishra National Rehabilitation University, Lucknow, Uttar Pradesh, India
*Author to whom correspondence should be addressed:
anjani_phys@yahoo.in (Anjani K. Pandey)
ABSTRACT
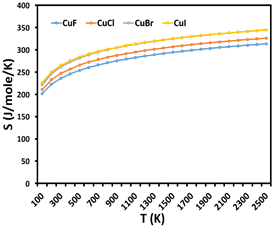
This paper presents a detailed investigation into the thermodynamic properties of copper monohalides (CuF, CuCl, CuBr, and CuI) based on spectroscopic data and partition function theory. Key thermodynamic quantities, including Gibbs free energy (G), enthalpy (H), entropy (S), and specific heat capacity at constant pressure (Cₚ), are calculated over a broad temperature range of 100 K to 3000 K. The analysis incorporates anharmonicity, nonrigidity, and stretching effects to improve the accuracy of the results. The relationship between temperature and these thermodynamic properties is explored through different modes of molecular motion, such as rotational, vibrational, and translational. Our results offer valuable insights into the behavior of copper monohalides, contributing to their application in fields like material science, nanotechnology, and industrial processes. These data are essential for designing copper-based compounds with optimized thermodynamic properties in advanced technological applications.
Significance of the study:
This study offers valuable insights into the thermodynamic behavior of copper monohalides (CuF, CuCl, CuBr, CuI) over a wide temperature range. By integrating spectroscopic data and partition function theory, it provides accurate calculations of essential properties like Gibbs free energy, enthalpy, and specific heat capacity. These findings are significant for fields such as material science and nanotechnology, enabling the design of copper-based compounds with optimized thermodynamic properties for advanced technological applications.
Summary of the study:
The paper investigates the thermodynamic properties of copper monohalides (CuF, CuCl, CuBr, CuI) using spectroscopic data and partition function theory. Thermodynamic quantities, including Gibbs free energy, enthalpy, entropy, and specific heat capacity, are calculated across a temperature range of 100 K to 3000 K. The study accounts for anharmonicity and nonrigidity effects, providing a deeper understanding of molecular motion. These results are essential for developing copper-based materials with optimized thermodynamic properties for use in nanotechnology and industrial processes.
