Anil Kumar, Harshitha D., C. G. Renuka
1 Department of Physics, Government First Grade College, Sindhanur-584128, India
2 P.G. Studies and Department of Physics, Shri Siddeshwar Government First Grade College, Nargund-582207, India
3 Department of Physics, Bangalore University, Bengaluru-560065, India
*Authors to whom correspondence should be addressed:
renubub@gmail.com (C. G. Renuka)
ABSTRACT
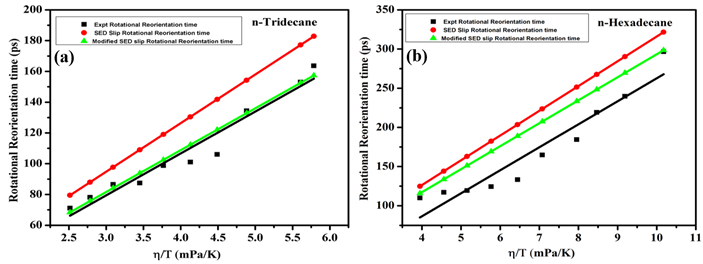
The rotational diffusion behavior of 3-(benzo[d]thiazol-2-yl)-7-(diethylamino)-2H-chromen-2-one (3BT7D2H-one) was investigated in two non-polar solvents, n-tridecane and n-hexadecane, across a range of temperatures. Steady-state and time-resolved fluorescence depolarization techniques were employed to measure the rotational reorientation times (τr) of the fluorescent probe molecule. The results demonstrate a linear dependence of τr on the viscosity-to-temperature ratio (η/T), highlighting that rotational dynamics are closely linked to changes in solvent viscosity. The experimental τr values were compared to predictions made by both the hydrodynamic Stokes-Einstein-Debye (SED) model and quasi-hydrodynamic models, including the Gierer-Wirtz (GW) and Dote-Kivelson-Schwartz (DKS) models. In both solvents, the observed τr values suggest sub-slip behavior, indicating that 3BT7D2H-one rotates with less frictional resistance than expected for a fully hydrodynamic regime. Specifically, the experimental τr values in n-hexadecane were found to be higher than those in n-tridecane, corresponding to the higher viscosity of the former solvent. While the SED model with sub-slip boundary conditions provided a reasonable approximation for the rotational times, both the GW and DKS models failed to quantitatively capture the experimental behavior. These models either underestimated or overestimated the frictional interaction between the solute and solvent, suggesting that additional factors such as solute-solvent size ratio and molecular shape might need to be incorporated for more accurate predictions. This study enhances the understanding of solute-solvent coupling in non-polar environments and underscores the limitations of traditional hydrodynamic models in describing molecular rotational diffusion in complex solvent systems.
Significance of the Study
This study provides important insights into the rotational dynamics of a non-polar fluorescent probe in n-alkane solvents, revealing how solvent viscosity influences molecular rotation. By comparing experimental data with traditional hydrodynamic and quasi-hydrodynamic models, the research highlights the limitations of these models in capturing the complex solute-solvent interactions. The findings emphasize the need for improved models that consider factors like solute-solvent size ratio and molecular shape, contributing to a deeper understanding of molecular dynamics in non-polar environments.
Summary of the Study
The study investigates the rotational diffusion of the fluorescent probe 3BT7D2H-one in non-polar solvents n-tridecane and n-hexadecane. Using fluorescence depolarization techniques, the rotational reorientation times (τr) were measured and compared with predictions from hydrodynamic (SED) and quasi-hydrodynamic (GW and DKS) models. The results showed a linear relationship between τr and the viscosity-to-temperature ratio (η/T). Although the SED model provided reasonable approximations, the GW and DKS models failed to accurately capture the experimental behavior, underscoring the need for refined models in complex solvent systems.
