Kiran B. Kore, Sandeep P. Kanade, Rahul Mahadeo Mendhe, Sandesh R. Jadkar, Musthafa Ottakam Thotiyl, Adinath M. Funde
1 Department of Physics, Savitribai Phule Pune University, Ganeshkhind, Pune – 411007. Maharashtra, India.
2 Department of Chemistry, Indian Institute of Science Education and Research (IISER)-Pune, Dr. Homi Bhabha Road, Pashan, Pune 411008, Maharashtra, India.
3 Centre for Energy Studies (Formerly School of Energy Studies), Savitribai Phule Pune University, Pune, Maharashtra, 411007, India.
*Author to whom correspondence should be addressed:
musthafa@iiserpune.ac.in (Musthafa Ottakam Thotiyl)
adinathf@gmail.com (Adinath Funde)
ABSTRACT
Sodium iron phosphate (NaFePO₄) has emerged as a promising cathode material for sodium-ion batteries (SIBs) due to its cost-effectiveness, environmental sustainability, and structural similarity to the well-established lithium iron phosphate (LiFePO₄) used in commercial lithium-ion batteries. The triphylite phase of NaFePO₄ offers a theoretical capacity of 154 mAh/g, making it an attractive candidate for large-scale energy storage applications. This study presents a scalable and economical synthesis route for producing triphylite NaFePO₄ through a two-step conversion process involving chemical delithiation of commercial LiFePO₄ followed by sodiation. Structural and morphological characterizations using X-ray diffraction (XRD), attenuated total reflectance (ATR) spectroscopy, and field-emission scanning electron microscopy (FESEM) confirmed the successful formation of phase-pure triphylite NaFePO₄ with an average crystallite size of 25 nm and flake-like morphology (~60 nm thickness). Electrochemical performance evaluation in half-cell configurations demonstrated a reversible capacity of 42 mAh/g after 100 cycles at 100 mA/g, with 91% capacity retention and near-100% Coulombic efficiency. Rate capability tests revealed stable performance across varying current densities (50–2000 mA/g), with capacity recovery to 91% upon returning to 50 mA/g. The low charge transfer resistance and structural stability of NaFePO₄ underscore its suitability for SIB applications. This work highlights a facile, scalable synthesis method that leverages existing LiFePO₄ infrastructure, offering a viable pathway for commercialization. The findings contribute to advancing sustainable and cost-efficient cathode materials for next-generation sodium-ion batteries.
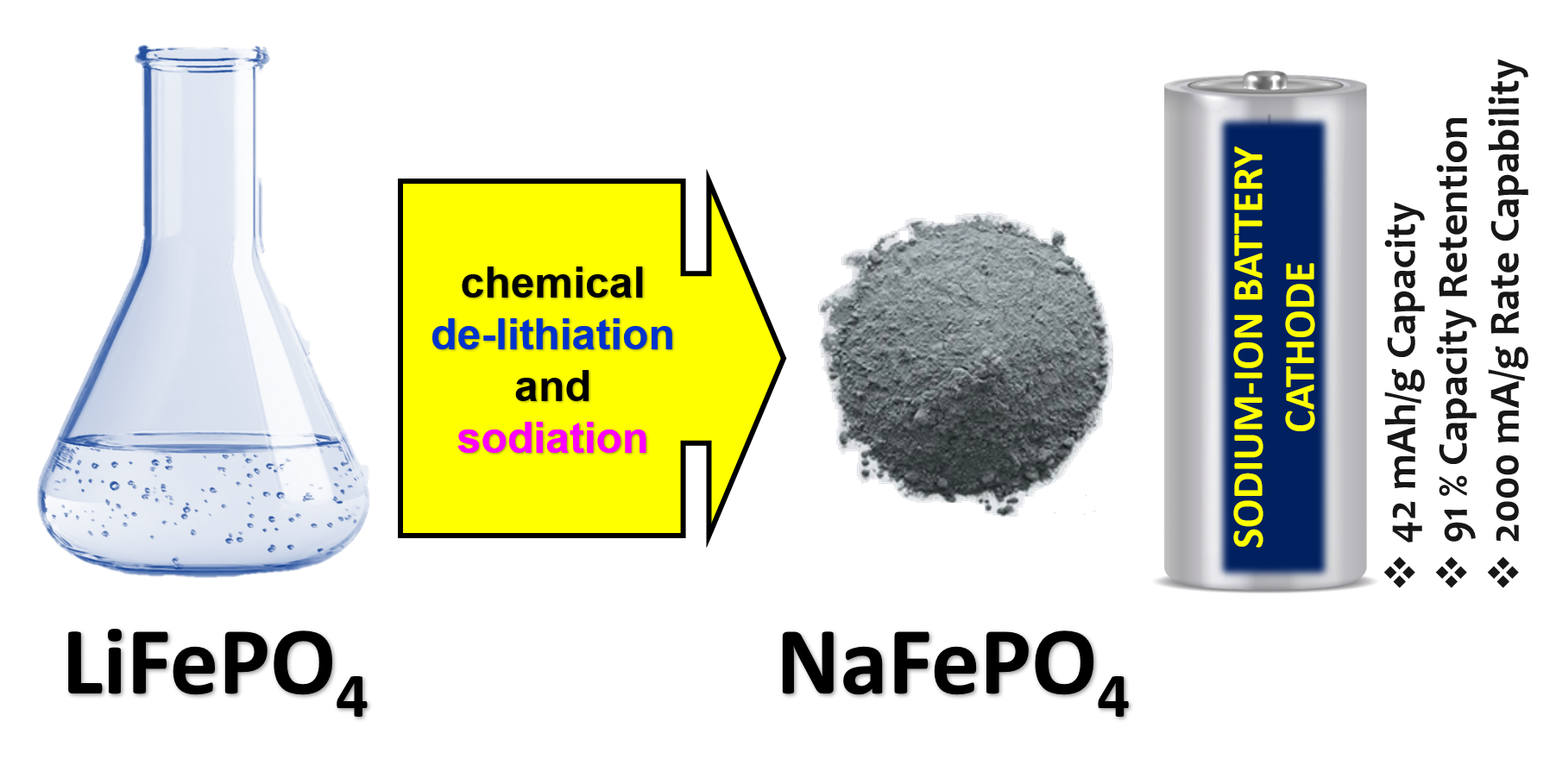
Significance of the Study:
The research addresses critical challenges in sodium-ion battery commercialization by proposing an economical, scalable cathode synthesis route. By utilizing abundant materials and simple aqueous processes, it offers a sustainable alternative to lithium-based systems, particularly for grid storage. The demonstrated structural stability and cycling performance underscore NaFePO₄/C’s potential to accelerate SIB adoption, bridging the gap between academic research and industrial deployment in renewable energy storage.
Summary of the Study:
This study demonstrates a cost-effective synthesis of triphylite NaFePO₄/C via chemical delithiation of commercial LiFePO₄, yielding phase-pure material with 25 nm crystallites and flake-like morphology. Electrochemical tests revealed stable cycling (91% retention after 100 cycles at 100 mA/g) and reversible capacities up to 42 mAh/g, despite kinetic limitations at high rates. The work establishes NaFePO₄/C as a viable SIB cathode, leveraging existing LiFePO₄ infrastructure for scalable production.
