Dnyaneshwar M. Sirsat, Hemant V. Chavan, Samadhan A. Shenmare, Dhiraj S. Mane, Satish M. Karape, Pravin S. Bhale
1 Department of Chemistry, Anandibai Raorane Arts, Commerce, and Science College, Vaibhavwadi, Dist- Sindhudurg-416 810, Maharashtra, India.
2 Department of Chemistry, A.S.P. College, Devrukh, Dist-Ratnagiri-415 804, Maharashtra, India.
3 Department of Chemistry, Yeshwantrao Chavan Mahavidyalaya, Tuljapur, Dist-Dharashiv-413 601, Maharashtra, India.
4 Department of Chemistry, Vishwasrao Naik Arts, Commerce, and Baba Naik Science Mahavidyalaya, Shirala, Dist-Sangli-415 408,, Maharashtra, India
*Author to whom correspondence should be addressed:
bhale.ps@gmail.com (P. S. Bhale)
ABSTRACT
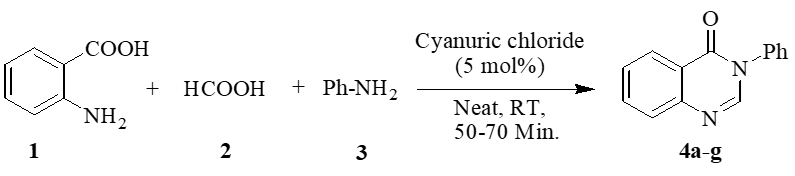
An efficient and environmentally benign one-pot synthesis of quinazolin-4(3H)-ones has been developed using 2,4,6-trichloro-1,3,5-triazine (TCT) as a cost-effective and commercially available catalyst under solvent-free conditions. This methodology involves a three-component reaction of anthranilic acid, diverse amines, and formic acid, proceeding smoothly in a single step without the need for intermediate isolation. The catalytic role of TCT significantly enhances reaction efficiency while maintaining mild conditions (room temperature), making the process both energy-efficient and operationally simple. Key advantages of this protocol include short reaction times (50–70 min), excellent product yields (88–95%), and broad substrate compatibility, accommodating both electron-donating and electron-withdrawing substituents on aromatic amines. The solvent-free approach aligns with green chemistry principles by eliminating hazardous organic solvents, reducing waste, and simplifying purification. Furthermore, the method avoids the use of expensive or moisture-sensitive reagents, addressing common limitations of traditional quinazolinone syntheses, such as harsh conditions, prolonged reaction times, and low yields. The synthesized quinazolin-4(3H)-ones are of significant pharmaceutical interest due to their diverse biological activities, including anticancer, antimalarial, and anti-inflammatory properties. This work not only provides a practical and scalable synthetic route but also highlights the potential of TCT as a versatile catalyst for multicomponent reactions. The combination of mild conditions, high atom economy, and environmental sustainability makes this protocol a valuable addition to the synthetic toolbox for medicinal and heterocyclic chemistry.
Significance of the Study:
This work provides a practical, scalable, and eco-friendly route to pharmacologically important quinazolinones, addressing key limitations of conventional methods (harsh conditions, low yields, and complex purification). The use of inexpensive TCT, solvent-free conditions, and room-temperature reactivity enhances industrial applicability. The synthesized derivatives, bearing modifiable functional groups, hold potential for drug discovery. The methodology’s simplicity, efficiency, and sustainability make it valuable for academic and pharmaceutical research, advancing green synthetic strategies in heterocyclic chemistry.
Summary of the Study:
This study presents an efficient, one-pot synthesis of quinazolin-4(3H)-ones via a three-component reaction of anthranilic acid, amines, and formic acid, catalyzed by 2,4,6-trichloro-1,3,5-triazine (TCT) under solvent-free conditions. The method offers excellent yields (88–95%), broad substrate scope, and operational simplicity at room temperature. TCT’s dual activation role enables mild, energy-efficient conditions while aligning with green chemistry principles by eliminating solvents and minimizing waste, making it a sustainable alternative to traditional approaches.
