Vaishnavi Bote, Bharati Shinde, Rupali Chavan, Aishwarya Jadhav, Rajesh Ghorpade, Shivaji Jamadade, Rahul Patil, Jyotiprakash Yadav, Ashok Chougale, Vishalkumar More
1 The New College Kolhapur, Shivaji University, Kolhapur-416206, Maharashtra, India.
2 Yashwantrao Chavan Mahavidyalaya, Halkarni, Shivaji University, Kolhapur-416206, Maharashtra, India.
3 Government Residence Women’s Polytechnic Tasgaon, Maharashtra, India.
4 Shri Yashwantrao Patil Science College Solankur, Shivaji University, Kolhapur-416206, Maharashtra, India.
5 USIC-Common Facility Centre, Shivaji University, Kolhapur-416206, Maharashtra, India.
*Author to whom correspondence should be addressed:
morevishalkumar@newcollege.ac.in (Vishalkumar More)
ashokdchougale@gmail.com (Ashok Chougale)
ABSTRACT
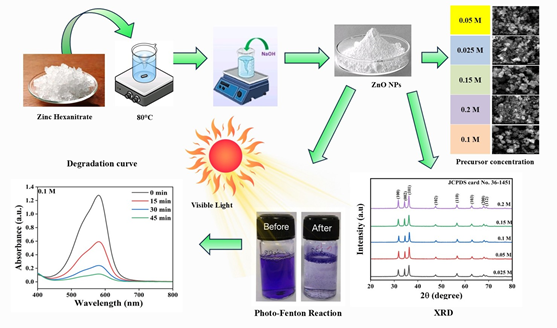
Water pollution poses a critical global challenge, necessitating advanced wastewater treatment solutions. Zinc oxide nanoparticles (ZnO NPs) have emerged as efficient catalysts for degrading organic pollutants via advanced oxidation processes. This study investigates the influence of precursor concentration on the morphology and catalytic efficiency of ZnO NPs synthesized via a cost-effective co-precipitation method. ZnO NPs were prepared using varying zinc nitrate hexahydrate concentrations (0.025 M, 0.05 M, 0.1 M, 0.15 M, and 0.2 M) and characterized using X-ray diffraction (XRD) and scanning electron microscopy (SEM). The XRD analysis confirmed the hexagonal wurtzite structure with high crystallinity, while SEM revealed morphological variations, including spherical and agglomerated nanostructures, depending on precursor concentration. The photocatalytic performance of ZnO NPs was evaluated in a visible-light-assisted photo-Fenton process for degrading crystal violet (CV) dye. Results demonstrated that ZnO NPs synthesized at 0.1 M precursor concentration exhibited superior catalytic activity, achieving 91.09% CV degradation within 45 minutes, compared to 75.90% (0.025 M), 88.87% (0.05 M), 85.24% (0.15 M), and 89.89% (0.2 M). Further optimization studies assessed the impact of catalyst dosage, initial dye concentration, and H₂O₂ levels. Increasing catalyst dosage from 30 mg/L to 90 mg/L enhanced degradation efficiency to 95.42%, while higher dye concentrations reduced catalytic performance due to active site saturation. An optimal H₂O₂ concentration of 0.1 M yielded maximum degradation, with excess H₂O₂ inhibiting the process by radical scavenging. This study highlights the critical role of precursor concentration in tailoring ZnO NP properties for efficient wastewater treatment, offering insights into scalable, eco-friendly dye degradation solutions.
Significance of the Study:
This work advances ZnO nanoparticle synthesis for efficient wastewater treatment by establishing precise precursor concentration control to optimize morphology and catalytic activity. The demonstrated 91.09% crystal violet degradation under visible light addresses key challenges in dye pollution remediation, offering a sustainable alternative to conventional methods. The systematic optimization of catalyst dosage, H₂O₂ levels, and dye concentration provides actionable insights for scaling solar-driven purification systems, particularly in textile industries. The findings contribute to nanotechnology-based environmental solutions with improved cost-effectiveness and performance.
Summary of the Study:
ZnO nanoparticles were synthesized via co-precipitation at varying precursor concentrations (0.025–0.2 M), with 0.1 M yielding optimal spherical morphology and 91.09% dye degradation. Photo-Fenton experiments revealed peak efficiency at 90 mg/L catalyst, 0.1 M H₂O₂, and 0.05 mM dye concentration. The study correlates nanoparticle crystallinity/morphology with catalytic performance, demonstrating visible-light-driven degradation mechanisms. This optimization framework enables tailored ZnO synthesis for industrial wastewater treatment, achieving 95.42% efficiency under ideal conditions while balancing economic and environmental factors.
