Rahul S. Salunke, Yogesh Nakate, Ahmad Umar, Amardip M. Patil, Umesh Nakate, Sotirios Baskoutas, Dhammanand J. Shirale
1 Nano-Structured Materials Processing Research Laboratory, Department of Electronics, Kavayitri Bahinabai Chaudhari, North Maharashtra University Jalgaon, MS 425001, India
2 Department of Chemistry, Faculty of Science and Arts, and Promising Centre for Sensors and Electronic Devices (PCSED), Najran University, Najran-11001, Kingdom of Saudi Arabia
3 Department of Materials Science and Engineering, The Ohio State University, Columbus 43210, OH, USA
4 Department of Chemistry, Kavayitri Bahinabai Chaudhari, North Maharashtra University Jalgaon, MS 425001, India
5 School of Semiconductor and Chemical Engineering, Jeonbuk National University, 567 Baekje-daero, Deokjin-gu, Jeonju 54896, Jeollabuk-do, Republic of Korea
6 Department of Materials Science, University of Patras, 26504 Patras, Greece
*Author to whom correspondence should be addressed:
djshirale@nmu.ac.in (Dhammanand J. Shirale)
ahmadumar786@gmail.com (Ahmad Umar)
ABSTRACT
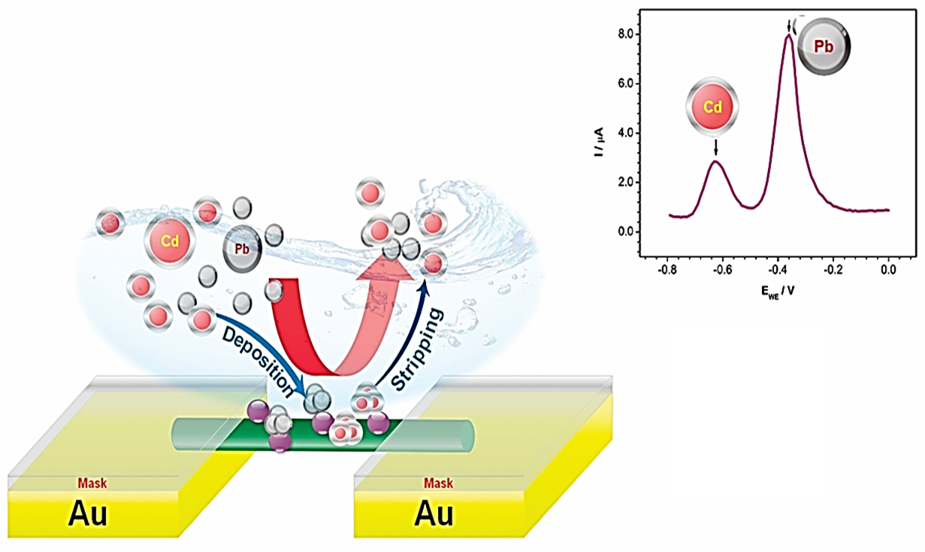
The fabrication and characterization of a high-performance, robust, and highly sensitive sensor based on a single polypyrrole nanowire (sPpy NW) decorated with cobalt oxide nanoparticles (CoOxNPs) for the selective recognition of lead (Pb2+) and cadmium (Cd2+) in potable water are described in this paper. The electrodeposition technique was used to prepare the sPpyNW/CoOxNPs assembly, and square wave anodic stripping voltammetry (SW-ASV) was used to assess sensor performance. Optimizing sensor performance, such as deposition time, deposition potential, and supporting electrolyte, was used to assess for best sensor performance. The developed sensor exhibited excellent linearity and the linear range for the detection of individual Cd2+ and Pb2+ ions was determined to be 0.00 to 0.11 μM and 0.00 to 0.12 μM, respectively. The detection limits observed for both Pb2+ and Cd2+ sensors are 0.22 μM (R2 = 0.9520) and 0.013 μM (R2 = 0.9723), respectively. The linear range for the detection of Pb2+ and Cd2+ sensors present in the same sample was determined to be 0.00 to 0.09 μM, with a detection limit of 0.026 μM (R2 = 0.8726) and 0.013 μM (R2 = 0.9468) for Pb2+ and Cd2+, respectively. The observed results revealed that the functionalization of CoOxNPs on the surface of a sPpyNW electrode seems to be a very effective way of developing a sensitive, selective, and stable electrochemical sensor for Pb2+ and Cd2+ions.
Significance of the study:
This study demonstrates a novel sensor using a polypyrrole nanowire decorated with cobalt oxide nanoparticles for detecting lead and cadmium in potable water. The high sensitivity, selectivity, and stability of the sensor underscore its potential for efficient environmental monitoring, ensuring safer drinking water and addressing critical public health concerns.
Summary of the study:
The paper presents the development and characterization of a sensor based on a polypyrrole nanowire decorated with cobalt oxide nanoparticles for lead and cadmium detection in water. Utilizing square wave anodic stripping voltammetry, the sensor achieved excellent sensitivity and selectivity, with detection limits of 0.22 μM for cadmium and 0.013 μM for lead, making it a promising tool for water quality monitoring.
