Vikas G. Patil, D. V. Mane
1 School of Sciences, Yashwantrao Chavan Maharashtra Open University, Nashik-422222, Maharastra, India
2 Department of Chemistry, Shri Chhatrapati Shivaji College Omerga, Dharashiv 413606, Maharastra, India
*Author to whom correspondence should be addressed:
vikasgpatil8@gmail.com (Vikas G. Patil)
ABSTRACT
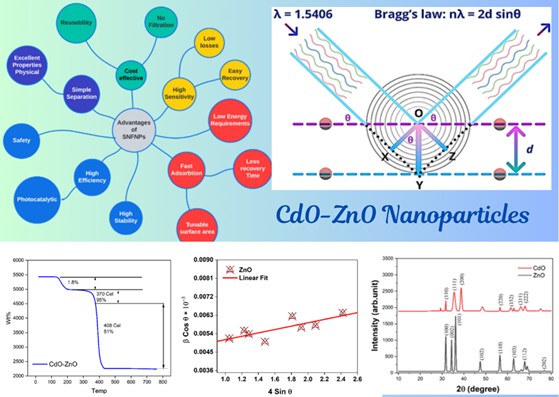
This study presents the ultrasonic-assisted sonochemical synthesis and comprehensive characterization of (CdO)₁₋ₓ(ZnO)ₓ mixed oxide nanoparticles (MONPs) using X-ray diffraction (XRD), Williamson-Hall (W-H) analysis, and thermogravimetric analysis (TGA). XRD patterns reveal distinct crystallographic phases, with CdO exhibiting cubic structure (Fm-3m) via prominent (110), (111), (200), (220), (152), (311), and (222) reflections, while ZnO adopts a hexagonal wurtzite structure (P6₃mc) with (100), (002), (101), (102), (110), (103), (112), and (202) planes. A secondary phase (*) confirms CdO-ZnO solid solution formation. Vegard’s law governs the composition-dependent lattice parameter variation, where pure CdO (a ≈ 4.695 Å) and ZnO (a ≈ 3.249 Å, c ≈ 5.206 Å) exhibit predictable shifts in MONPs (e.g., Cd₀.₅Zn₀.₅O yields a ≈ 4.5 Å for cubic or expanded hexagonal parameters). Williamson-Hall analysis quantifies strain and crystallite size effects, demonstrating increased tetrahedral (LA) and octahedral (LB) site distances with rising lattice constant due to atomic size disparity. TGA profiles indicate a two-stage decomposition: an initial 1.8% mass loss (370°C, moisture/solvent removal) followed by a major 51% loss (408°C, organic precursor degradation), culminating in 47.2% residual mass at 800°C, affirming high thermal stability. These findings underscore the structural integrity and tunable properties of CdO-ZnO MONPs, positioning them as robust candidates for high-temperature applications, including photocatalysis, gas sensing, and optoelectronic devices.
Significance of the Study:
This study advances the design of CdO-ZnO mixed oxide nanoparticles (MONPs) by elucidating composition-dependent structural and thermal stability. The lattice parameter tunability via Vegard’s law enables tailored material properties for optoelectronics and catalysis. Demonstrated thermal resilience underscores suitability for high-temperature applications, such as energy storage or sensors. The coexistence of cubic and hexagonal phases in MONPs highlights their multifunctionality, bridging structural diversity with enhanced performance. These insights provide a blueprint for engineering thermally robust, compositionally adaptable oxides for next-generation technologies.
Summary of the Study:
This study synthesizes (CdO)₁₋ₓ(ZnO)ₓ mixed oxide nanoparticles (MONPs) and characterizes their structural and thermal properties. XRD confirms cubic CdO (a≈4.695 Å) and hexagonal ZnO (a≈3.249 Å, c≈5.206 Å), with MONPs exhibiting composition-dependent lattice parameters adhering to Vegard’s law. Ion spacing in tetrahedral (LA) and octahedral (LB) sites increases with lattice expansion. TGA reveals high thermal stability (47.2% residual mass at 800°C) after organic decomposition. Tailorable composition and robustness position MONPs for photocatalysis, sensors, and energy storage, highlighting their promise as advanced functional materials.
