Suneel, Neda Tabassum, Devendra Pratap Mishra, Moonish Aftab
1 Department of Chemistry, Integral University, Lucknow, Uttar Pradesh-226026, India.
2 Government Engineering College, AICTE, Dr. A. P. J. Abdul Kalam Technical University, Lucknow, Uttar Pradesh-226026, India.
3 Department of Botany, University of Kashmir, Srinagar-190006, Kashmir, India
* Author to whom correspondence should be addressed:
suneelphd@student.iul.ac.in (Suneel)
ABSTRACT
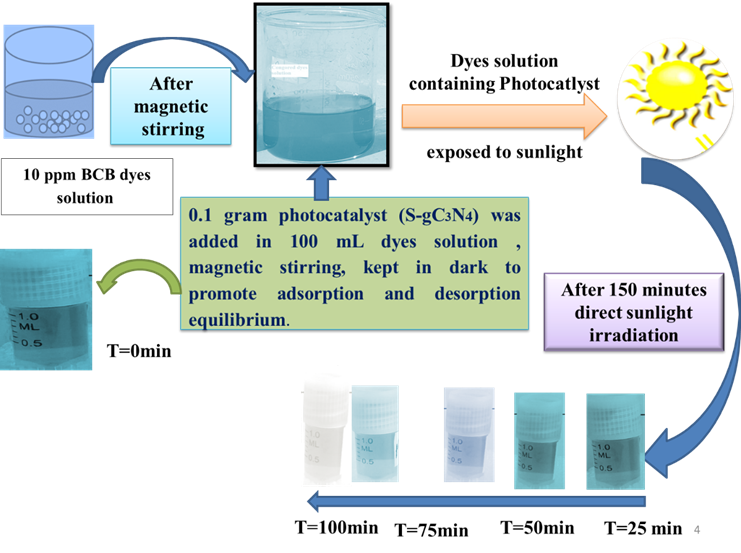
This study presents a facile thermal polymerization method for synthesizing sulfur-doped graphitic carbon nitride (S-g-C₃N₄) using thiourea as a precursor and evaluates its photocatalytic efficiency in degrading hazardous organic dyes—Brilliant Cresyl Blue (BCB) and Malachite Green (MG)—under natural sunlight irradiation. The structural and functional properties of the synthesized S-g-C₃N₄ were characterized using X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR), confirming its high crystallinity and successful sulfur incorporation. XRD analysis revealed characteristic peaks at 13.07° and 27.42°, corresponding to the (100) and (002) planes of graphitic carbon nitride, while FTIR spectra exhibited prominent absorption bands at 3153 cm⁻¹ (N-H stretching), 1636–1240 cm⁻¹ (C-N heterocycles), and 805–811 cm⁻¹ (tri-s-triazine units). The photocatalytic performance demonstrated degradation efficiencies of 64.86% for BCB and 81.28% for MG within 100 minutes of solar irradiation, following pseudo-first-order kinetics with rate constants of 0.01077 min⁻¹ and 0.01734 min⁻¹, respectively. The half-life times for BCB and MG degradation were calculated as 64.36 min and 39.97 min, indicating rapid dye mineralization. The enhanced photocatalytic activity of S-g-C₃N₄ is attributed to improved charge separation and visible-light absorption due to sulfur doping. This work highlights the potential of S-g-C₃N₄ as an eco-friendly, cost-effective photocatalyst for wastewater treatment, offering a sustainable solution for mitigating textile dye pollution under solar irradiation.
Significance of the Study:
This study demonstrates the efficient solar-light-driven photocatalytic degradation of hazardous dyes (Brilliant Cresyl Blue and Malachite Green) using sulfur-doped graphitic carbon nitride (S-g-C₃N₄). The work highlights a facile, scalable synthesis method for S-g-C₃N₄ with enhanced visible-light activity, offering a sustainable solution for wastewater treatment. The findings contribute to the development of metal-free, cost-effective photocatalysts, addressing critical environmental challenges posed by textile industry effluents while reducing reliance on energy-intensive purification methods.
Summary of the Study:
S-g-C₃N₄ was synthesized via thermal polymerization of thiourea and characterized using FTIR and XRD. The photocatalyst exhibited 64.86% and 81.28% degradation of Brilliant Cresyl Blue and Malachite Green, respectively, under solar irradiation, following pseudo-first-order kinetics. Enhanced activity was attributed to sulfur doping, which improved charge separation and light absorption. The study presents a promising, eco-friendly approach for dye wastewater remediation, emphasizing the potential of S-g-C₃N₄ for large-scale environmental applications under natural sunlight.
